WATER POLLUTION
Water pollution is the contamination of water bodies (e.g. lakes, rivers, oceans, aquifers and groundwater). This form of environmental degradation occurs when pollutants are directly or indirectly discharged into water bodies without adequate treatment to remove harmful compounds.
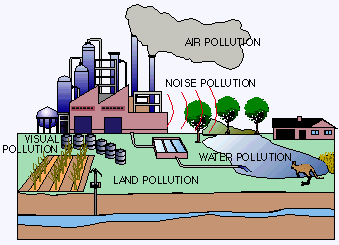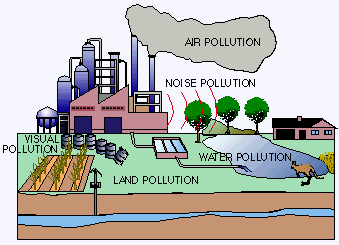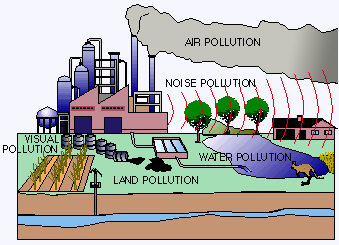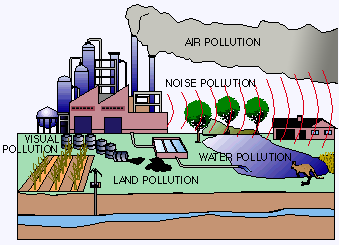 Water pollution affects the entire biosphere – plants and organisms living in these bodies of water. In almost all cases the effect is damaging not only to individual species and population, but also to the natural biological communities.
Water pollution affects the entire biosphere – plants and organisms living in these bodies of water. In almost all cases the effect is damaging not only to individual species and population, but also to the natural biological communities.Introduction
Water pollution is a major global problem which requires ongoing evaluation and revision of water resource policy at all levels (international down to individual aquifers and wells). It has been suggested that water pollution is the leading worldwide cause of deaths and diseases, and that it accounts for the deaths of more than 14,000 people daily. An estimated 580 people in India die of water pollution related illness every day. About 90 percent of the water in the cities of China is polluted. As of 2007, half a billion Chinese had no access to safe drinking water. In addition to the acute problems of water pollution in developing countries, developed countries also continue to struggle with pollution problems. For example, in the most recent national report on water quality in the United States, 45 percent of assessed stream miles, 47% of assessed lake acres, and 32 percent of assessed bays and estuarine square miles were classified as polluted.The head of China's national development agency said in 2007 that one quarter the length of China's seven main rivers were so poisoned the water harmed the skin.
Water is typically referred to as polluted when it is impaired by anthropogenic contaminants and either does not support a human use, such as drinking water, or undergoes a marked shift in its ability to support its constituent biotic communities, such as fish. Natural phenomena such as volcanoes, algae blooms, storms, and earthquakes also cause major changes in water quality and the ecological status of water.The specific contaminants leading to pollution in water include a wide spectrum of chemicals, pathogens, and physical changes such as elevated temperature and discoloration. While many of the chemicals and substances that are regulated may be naturally occurring (calcium, sodium, iron, manganese, etc.) the concentrationis often the key in determining what is a natural component of water and what is a contaminant. High concentrations of naturally occurring substances can have negative impacts on aquatic flora and fauna.
Oxygen-depleting substances may be natural materials such as plant matter (e.g. leaves and grass) as well as man-made chemicals. Other natural and anthropogenic substances may cause turbidity (cloudiness) which blocks light and disrupts plant growth, and clogs the gills of some fish species.
Many of the chemical substances are toxic. Pathogens can produce waterborne diseases in either human or animal hosts. Alteration of water's physical chemistry includes acidity (change in pH), electrical conductivity, temperature, and eutrophication. Eutrophication is an increase in the concentration of chemical nutrients in an ecosystem to an extent that increases in the primary productivity of the ecosystem. Depending on the degree of eutrophication, subsequent negative environmental effects such as anoxia (oxygen depletion) and severe reductions in water quality may occur, affecting fish and other animal populations.
Transport and chemical reactions of water pollutants
Most water pollutants are eventually carried by rivers into the oceans. In some areas of the world the influence can be traced one hundred miles from the mouth by studies using hydrology transport models. Advanced computer models such asSWMM or the DSSAM Model have been used in many locations worldwide to examine the fate of pollutants in aquatic systems. Indicator filter feeding species such as copepods have also been used to study pollutant fates in the New York Bight, for example. The highest toxin loads are not directly at the mouth of the Hudson River, but 100 km (62 mi) south, since several days are required for incorporation into planktonic tissue. The Hudson discharge flows south along the coast due to the coriolis force. Further south are areas of oxygen depletion caused by chemicals using up oxygen and by algae blooms, caused by excess nutrients from algal cell death and decomposition. Fish and shellfish kills have been reported, because toxins climb the food chain after small fish consume copepods, then large fish eat smaller fish, etc. Each successive step up the food chain causes a cumulative concentration of pollutants such asheavy metals (e.g. mercury) and persistent organic pollutants such as DDT. This is known as bio-magnification, which is occasionally used interchangeably with bio-accumulation.
Large gyres (vortexes) in the oceans trap floating plastic debris. The North Pacific Gyre, for example, has collected the so-called "Great Pacific Garbage Patch", which is now estimated to be one hundred times the size of Texas. Plastic debris can absorb toxic chemicals from ocean pollution, potentially poisoning any creature that eats it. Many of these long-lasting pieces wind up in the stomachs of marine birds and animals. This results in obstruction of digestive pathways, which leads to reduced appetite or even starvation.
Groundwater pollution is much more difficult to abate than surface pollution because groundwater can move great distances through unseen aquifers. Non-porous aquifers such as clays partially purify water of bacteria by simple filtration (adsorption and absorption), dilution, and, in some cases, chemical reactions and biological activity; however, in some cases, the pollutants merely transform tosoil contaminants. Groundwater that moves through open fractures and caverns is not filtered and can be transported as easily as surface water. In fact, this can be aggravated by the human tendency to use natural sinkholes as dumps in areas of karst topography.
There are a variety of secondary effects stemming not from the original pollutant, but a derivative condition. An example is silt-bearing surface runoff, which can inhibit the penetration of sunlight through the water column, hampering photosynthesis in aquatic plants.
Measurement
Water pollution may be analyzed through several broad categories of methods: physical, chemical and biological. Most involve collection of samples, followed by specialized analytical tests. Some methods may be conducted in situ, without sampling, such as temperature. Government agencies and research organizations have published standardized, validated analytical test methods to facilitate the comparability of results from disparate testing events.
